Ultrasonic welders are used to assemble a variety of plastic products. However, the requirements for a welder to make medical devices are much stricter than those for a machine to weld, say, toys or consumer products. Here’s a list of things to think about:
Secure data collection, analysis and transfer
The most critical criterion for an ultrasonic welding system used in medical device manufacturing is the ability to support the secure capture of assembly data, when such data is used to comply with global regulatory requirements, including those of the U.S. Food and Drug Administration (FDA) and the European Union’s Medical Device Regulation. For example, FDA’s 21 Code of Federal Regulations (CFR) Part 11 for medical devices requires weld systems to validate users for system security, securely store weld data for individual products, create non-editable audit trails, and deliver data that meets traceability standards (unique device identifier or UDI).
UDI requirements extend to all forms of manufacturing data: data that provides a complete audit trail; data that can trace finished devices individually or by lot or batch; data about component parts; and data about the quality of individual ultrasonic welds, right down to the day, time and operator in charge.
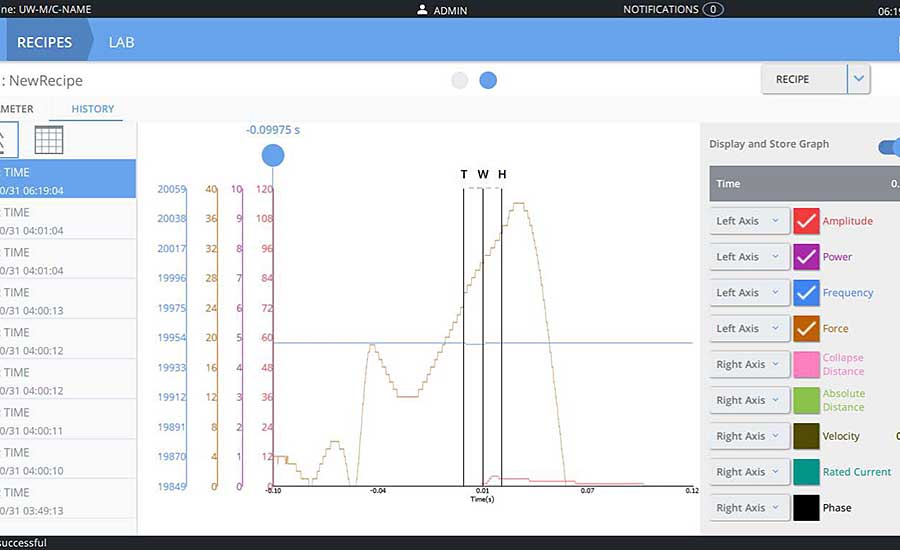
Ultrasonic welders for medical device assembly must be able to securely capture data on the quality of every joint, right down to the day, time and operator in charge. Photo courtesy Branson Welding and Assembly at Emerson.
The secure transfer of that data starts with the ability to authenticate the data recipient—whether an enterprise, regulator or customer—and then securely transfer the data. Of course, such security protects not only product-data transfers, but also in-field system software upgrades, remote data analysis, and remote system troubleshooting and diagnostics.
A more immediate use of weld data involves in-house quality control. Real-time data monitoring allows for statistical process control using closed-loop controls. Monitoring weld parameters enables manufacturers to maintain high production speeds, quality level and yields.
Scale-up and automation of validated assembly processes
Medical assembly processes require not only the successful joining of components, but also the ability to validate the process and scale it up to full production. To simplify validation and eventually reach large-scale production, you will want to start with ultrasonic equipment that is built around a common, configurable architecture or platform. A well-chosen welding platform combines scalable technology with a platform design that permits mounting the weld actuator in a variety of configurations (top, horizontal, inverted, front or rear) to maximize the factory floor or clean room footprint. Top platforms also offer the computing power, software and communications capabilities required to support full automation.

The GSX-E1 ultrasonic welder is certified for use in ISO class 5.5 clean rooms. Photo courtesy Branson Welding and Assembly at Emerson.
By adopting the right platform to launch your product development process, a joining process or recipe that is validated on a small scale in a laboratory can be directly translated out, in scaled-up form, to a clean room or a manufacturing floor. Developing and scaling up on the same platform—power supply, actuator, stack, data collection and controls—can dramatically simplify the transfer of key weld parameters and speed up the validation of automated assembly processes whenever expansion is required.
Microwelding and force controls
The need to miniaturize wearable or implanted medical devices means that their component parts keep getting smaller, lighter and more delicate. Many of these parts also incorporate more sophisticated sensors and electronics and require assembly to ever-tighter tolerances. Meeting these dual needs has given rise to the “microwelding” trend. To ensure that your ultrasonic welding equipment can meet current and future requirements involving extremely small, thin-walled or electronics-laden components, you will need welders with precise, multimode weld and force controls.
Advances in ultrasonic welders allow for precise regulation of downforce through the use of computer-controlled electromechanical actuators. Also helpful are new technologies such as “force stepping” (programmable adjustments in force as the weld proceeds) and dynamic weld modes that can monitor, recalculate and adjust multiple weld parameters (force, weld energy, velocity, distance) in real time to optimize weld performance within a user-specified target result, such as part compression, positioning and pull strength. Technological advances like these dramatically expand the capabilities of ultrasonic welders, giving manufacturers the ability to assemble and join components in accordance with a design profile instead of a single fixed weld parameter.
Sustainability
By choosing ultrasonic welding equipment that meets these requirements, you will be able to maximize the sustainability of your process. Ultrasonic welding offers exceptional energy efficiency, and, when properly optimized, the process offers high yields and a minimum of flash or waste. Further, since the process creates permanent, part-to-part plastic welds without the use of adhesives, fasteners or other consumables, the supply chain is simplified.
Flexibility
For manufacturers that build to order or have a high mix of production, the ability to reconfigure equipment for production changeovers is vital. To meet this need, select ultrasonic welding systems that offer digital controls to store and replicate programmable production recipes.


