Fastening or Pressing of Medical Devices
For a wide range of medical devices, fastening or pressing is often the best assembly method

One medical device manufacturer uses a toggle press to cut 40 to 55 discs per minute from a silicon strip using a cookiecutter- like die. Photo courtesy Schmidt Technology Corp.

Auto-feed screwdriving machines ensure accurate screw location during medical device assembly. Photo courtesy Design Tool Inc.

Workers use handheld and stationary low-torque screwdrivers to assemble hearing aids and other tiny medical devices. Photo courtesy DEPRAG

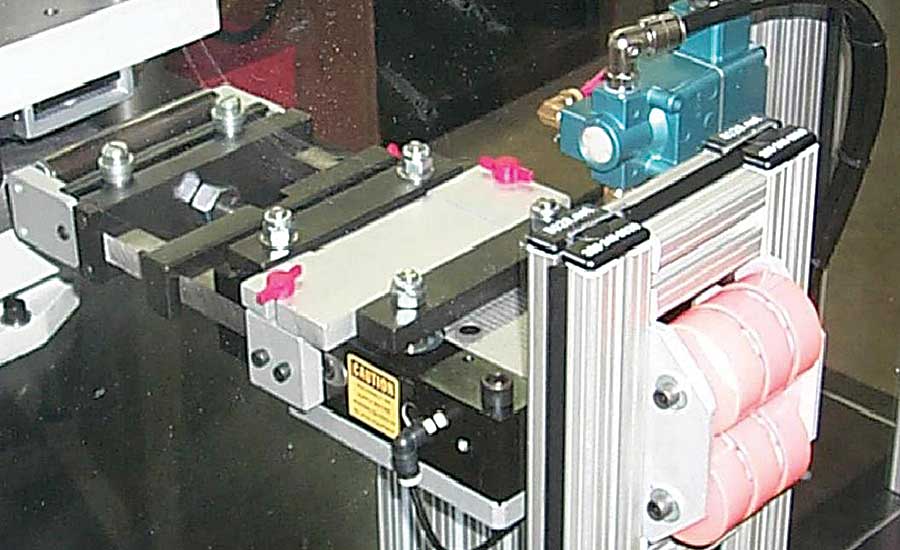
Custom tooling and a low-pressure riveter enable one manufacturer to radially form a small rivet (less than 1 millimeter in diameter) for an endoscopic clamp. Graphic courtesy BalTec Corp.

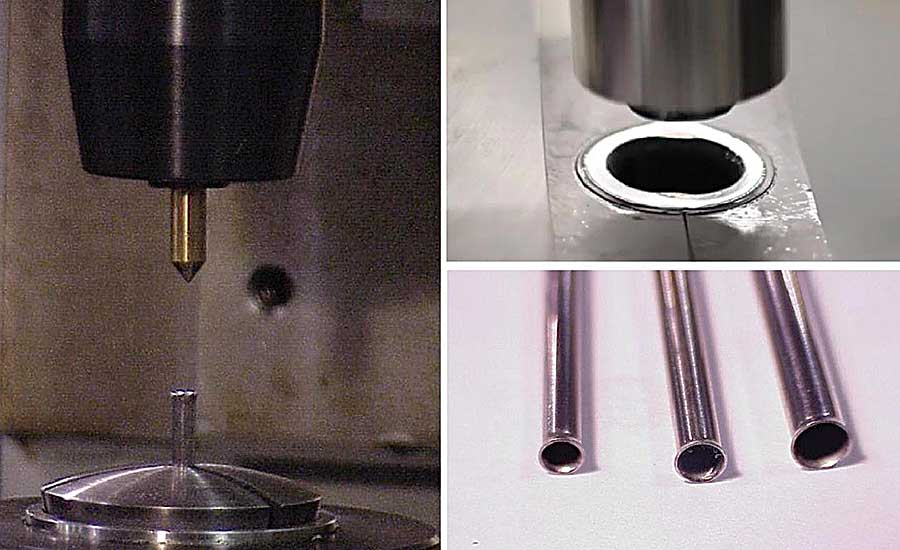
Orbital and radial forming is a better way to produce catheters with a flared end than a standard press. Photos courtesy Orbitform LLC





Fastening and pressing equipment have been used to assemble medical devices for decades, and that’s not likely to change anytime soon. Screwdrivers, for example, are regularly used to install screws in products as diverse as hospital beds, joint implants and hearing aids. Presses are even more versatile, with manufacturers using them to insert plungers into syringes, markerrivets into stents and tiny rivets into scissors at the end of endoscopic instruments. As the following case histories show, both processes enable manufacturers to meet these and other assembly challenges.
Cutting Compact (Silicon) Discs
Several medical devices feature subassemblies that contain silicon discs. For the last few years, one medical device manufacturer has used a Model No. 34.12 toggle press from Schmidt Technology Corp. to cut 40 to 55 discs per minute from a silicon strip using a steel rule die (also known as a cookie-cutter die). The press also simultaneously punches holes and slits inside each disc.
Dave Zabrosky, North American sales manager for Schmidt, says the machine cuts discs that are 12 millimeters in diameter by 2 millimeters thick, and 0.5 inch in diameter by 0.1 inch thick. Each punched hole has a diameter of 0.06 inch.
The press is housed in a selfcontained workstation that features all-around guarding and is controlled by a PLC. According to Zabrosky, the control system allows for fully automated, single operation and setup modes. Cycle rate is based on a delay timer setting.
An external motorized reel feeds the silicon strip into the cutting die in 0.9- or 0.545-inch sections. After the pneumatic press pushes the material strip through the die, discs and scrap stay on a polycarbonate carrier, and small-hole slugs drop into a catch bin. The processed strip is then automatically moved onto a takeup product reel, where the operator removes the strip.
In fully automated mode, the operator loads the material, closes the guarding and initiates the machine. It stops either when material runs out (via a sensor) or by the operator.
In the single-operation mode, the operator presses the start button to initiate stripping and cutting. This mode is also used to clear the machine of material at the end of a run.
Setup mode is for machine troubleshooting and changing the feeder or die. The latter task takes less than 5 minutes and requires no hand tools.
Presentation is Always Important
Successful manufacturers of medical devices and equipment know it’s important to always present their products in the best light, both aesthetically and functionally. They also know, thanks to Design Tool Inc. (DTI), the importance of properly presenting screws during assembly to optimize productivity and joint quality.
DTI’s medical industry customers include some of the largest in the industry: Atrion Medical, Stryker, Hill Rom and Carestream Health. Vic Glenn, president of DTI, says some use DTI’s screw presenters, while others use the supplier’s auto-feed screwdriving machines to build high-performance hospital beds and rails, X-ray display screens, plastic pumps, wheelchair assemblies, stretchers, walkers and humidifiers.
According to Glenn, screw presenters are used in fastener applications that are not suitable for auto-feed screwdriving equipment. These include hardto- reach locations and when the fastener length-to-head-diameter ratio is 1-to-1 or less. In these cases, the screw will tumble through a feed tube and not have the correct orientation when it reaches the screwdriver.
DTI’s presenters properly orient and present a screw to a screwdriver equipped with either a magnetic bit or a vacuum pickup attachment. Glenn says that manufacturers like the presenters because they can be used with both pneumatic and DC drivers. They also like that the magnetic bit and vacuum pickup attachment eliminate the possibility of fasteners falling into the assembly.
“Our auto-feed machines are custom built for each medical device application to ensure easy operation and accurate screw location,” explains Glenn. “They feed and drive screws for applications with deep counterbores, minimal screw head clearance and hard-to-reach locations.”
Machines used with DC screwdrivers only feed the next screw when the fastening process is within specification. If the fastening process is not successful, the operator is alerted that the screw is not tightened properly. He can then remove or retighten the fastener before the next screw is fed to the screwdriver.
Syringe Assembly and Function Testing
Process monitoring is a standard assembly practice for medical manufacturers. One company, for example, recently began assembling its syringes with an Electro-Mechanical Assembly Press (EMAP) from Promess Inc. that also provides force-vs.-position monitoring.
“The manufacturer had serious problems with the original syringe assembly technique, says Glenn Nausley, president of Promess. “Due to no process monitoring and very little quality control, rubber seals on the end of the plunger would sometimes become raised, rolled or offcenter. This problem often made the syringe difficult to use, or unusable. Plus, endof- line testing doesn’t catch a faulty assembly until additional processes have been completed on the part, adding cost to a bad part.”
The company analyzed the situation, and found that the problem was due to an air-actuated cylinder used in the assembly process. Its force could not be precisely controlled to accurately insert the plunger into the syringe. After trying to control the press in a different, but unsuccessful, way, the company reached out to Promess.
Within a short time, Promess application engineers determined the exact force at which the EMAP inserts the plunger into the syringe. Closed-loop feedback (from a built-in load cell) provides immediate force-vs.-position monitoring during pressing to ensure that the same force level (±0.5 percent) is applied every time. An encoder in the press’s motor keeps its positional accuracy within ±25 microns.
According to Nausley, the company is now able to verify correct assembly of every syringe, and perform a function test in the same station. This setup not only eliminates the need for end-of-line testing. It also gives the manufacturer 100 percent part traceability.
High-Precision Riveting
Orbital and radial forming, or riveting, may be mature technologies, but they haven’t remained static. Engineers who typically think of orbital and radial riveting for assembling robust automotive parts need to know that these cold-forming technologies are also effective for small, precision applications, such as medical devices.
One medical device manufacturer is using a servo-driven orbital riveter from Orbitform LLC to rivet small scissors at the end of endoscopic surgical instruments. The joints for these tools pivot on a small stainless steel rivet (0.058-inch diameter) that must be headed to a tight tolerance.
Prior to contacting Orbitform, the company often heard from surgeons about inconsistency in the feel of the tools during usage. The challenge was to come up with a way to form the rivet in the scissors assembly so that the opening and closing of the blade had a consistent feel.
“Due to the stack-up tolerances within the parts in the assembly, conventional riveting to a positive stop was not an option,” says Bryan Wright, vice president of sales at Orbitform. “Instead, we developed a Process Intelligence (process monitoring) method to form the assembly to a specific clamp load. This involved placing a load cell in the riveter’s fixture nest to capture the load force.”
During assembly, the scissors are held stationary, and the riveter advances up from the bottom to form the rivet until the proper clamp load force is achieved. The end result is a pair of scissors with a repeatable opening and closing force that is well within the customers’ required range.
The Big Challenge of Miniaturization
Consumer products continue to get smaller, and medical devices are not exempt from this trend. A good example is hearing aids. Many tiny models can be positioned either behind the ear or inside the ear canal.
A couple of years ago, DEPRAG developed the Nanomat screwdriver that many manufacturers use to assemble hearing aids and other tiny medical devices. Available in both handheld and stationary versions, the screwdriver only produces from 8 to 300 newtonmicrometers of torque, with speeds of up to 2,000 rpm.
Four torque ranges are possible, all of which are applied with a high degree of precision. According to Lori Logan, marketing manager at DEPRAG, hearing aids generally require a torque as low as 10 newton-micrometers, and the Nanomat can achieve that either as a handheld or a stationary unit integrated into one of DEPRAG’s automatic assembly machines.
DEPRAG also makes semiautomatic assembly stations. Logan says one eyeglasses manufacturer uses a station that includes a Micromat EC screwdriver, a process controller, a screwfeeder, a position control stand and electronic measurement controllers. Assembly takes only a few seconds, and involves fastening the arms of the glasses onto the frame with tiny screws that are barely visible to the naked eye.
The screwdriver is the size of a ballpoint pen, but designed to meet industrial demands. Equally important, its torque, rotation angle, speed, standby timing and direction of rotation can be individually programmed (within its performance range) for each screwdriving task.
Neither Fitting nor Forming is a Pressing Problem
Two of the more interesting medicaldevice assembly applications that press and riveting equipment supplier BalTec Corp. has worked on involve press-fitting handheld laparoscopic endoscopes and radially forming a rivet for an endoscope clamp. Each project is for a different manufacturer, notes Chuck Rupprecht, vice president and general manager at BalTec.
For the press-fitting application, the company required all gripping-device parts to have a tight fit and align at the proper height, and the final assembly to have no markings. BalTec provided a custom workcell built around a direct-acting DA-850 pneumatic press that produces up to 8.5 kilonewtons of force and has an 80-millimeter stroke.
“Custom upper press tooling and a die-set ensure down stroke accuracy,” says Rupprecht. “A load cell mounted on the press ram (within the die set), and a distance sensor mounted on the press cylinder measure press force and stroke distance during press fitting.”
The press’s process monitoring system, in turn, compares these values with established tolerances. Audio sounds and visible letters (OK or NOK) indicate whether the pres fit is acceptable or not. To prevent part marking, a custom fixture is treated with nonabrasive material.
Rupprecht says radially forming the endoscopic clamp rivet is a bit challenging due to its small size (less than 1 millimeter in diameter). To properly form the rivet, BalTec developed a workcell featuring its RN-181R low-pressure riveter with custom form tooling and fixturing. The riveter has a 30-millimeter stroke.
“An HPP-25 process controller with force and distance sensors is mounted to the riveter and provides data logging and total signature analysis of each formed rivet,” explains Rupprecht. “It establishes specific height and low forming-force (less than 1.5 kilonewtons) tolerances to prevent shank swelling of the rivet during installation, and to ensure proper pivot actuation of the rivet during endoscopic procedures.”
Implant Fastener Keeps Boomers Moving
Today’s baby boomers tend to live more active lives than those in the past. This fact has some medical device companies, like Skeletal Dynamics, developing implants to withstand up to 1 million load cycles without loosening or backing out, rather than the traditional 100,000. One such implant is the Align Radial Head System (ARHS), which features an artificial elbow joint designed to restore the natural function of the native radial head.
Older prosthetic radial heads featured one of two designs, both of which had drawbacks. The traditional fixed monoblock design offered stability, but could not be aligned to the patient’s anatomy and tended to wear away natural tissue such as cartilage.
The bipolar radial head did align with the patient’s native anatomy by rotating in a polyethylene sheath, but would not remain in the correct position because it would not lock. To ensure the ARHS implant stays locked, Skeletal Dynamics specifies that surgeons use Spiralock nuts or inserts having a 30-degree wedge ramp cut at the root of the female thread, instead of the usual 60-degree cut.
Initially, Skeletal Dynamics was hesitant to specify Spiralock components due to concerns about the manufacturability of the self-locking thread. But, Spiralock Corp. allayed those fears by making prototype tooling, customizing tools to cut the thread form and working closely with their contract manufacturer to increase production.
When the ARHS implant is surgically installed in a patient, proprietary instrumentation allows alignment of the radial head as it would be in the patient’s native anatomy. Once the device is oriented in this natural position, the surgeon locks the device in place by tightening the Spiralock setscrew (made of cobalt chrome) against a long titanium stem for three-point fixation.
Under clamp load, the crests of the threads on any standard male bolt are drawn tightly against the wedge ramp. This eliminates sideways motion that causes vibrational loosening and distributes the threaded joint’s load throughout all engaged threads. The load percentage on the first engaged thread is low, reducing the chance of bolt failure and improving product performance.
Installing Markers Into Stents
Schmidt offers a wide variety of toggle presses. One of them, the 13RF, is used by a medical device manufacturer to insert four tiny marker-rivets into eyelets of a cobalt-chromium stent.
An operator loads a marker-rivet (0.027-inch in diameter) into the nest, lays the stent into a V-block for support, and places an eyelet over the markerrivet. He pulls down the press handle, allowing the upper forming tool to contact the marker-rivet and form it to a fixed position in the eyelet. The operator then lifts the V-block and repositions the stent for the next marker-rivet installation.
“A high degree of accuracy is required from the tooling side in this application,” explains Zabrosky. “The tooling locates and supports one marker and the stent relative to each other. It also helps the operator manipulate the stent while positioning it for installation of the four marker-rivets.”
A Flare for Different Devices
Producing catheters with a flared end is more challenging than making those with a straight end. This reality led one catheter manufacturer to ask Orbitform if its orbital and radial technology could flare the end of catheters without cracking the tubing. Wright says the manufacturer reached out a few years ago after reading an application bulletin on Orbitform’s website.
“The customer was press flaring the end of the tube, and this method was causing the material to crack due to excessive force being applied to the part,” says Wright. “Orbital and radial forming, in contrast, induces 80 percent less forming force into the part than the press process.”
After Orbitform’s solution lab successfully processed several sample catheters, the company replaced its press with Orbitform’s B-125 benchtop orbital riveting machine. It features an orbital head and six quick-change fixture blocks designed to handle parts of all sizes.
The manufacturer also integrated the machine with Process Intelligence, Orbitform’s proprietary process monitoring technology. Process Intelligence verifies rivet presence and monitors forming force, pressure, ram stroke distance and other parameters to immediately detect when a catheter is outside the acceptable tolerance range.
Another manufacturer is using a customized B-500 benchtop orbital and radial riveting machine to form rivets in multiple parts, including a surgical stapler. Previously, the company formed each part on a different machine.
The key to the B-500’s flexibility is its ability to accept interchangeable multispindle, multi-point or Parallel Plate Eccentric Drive forming heads. The latter type enables peen tools to simultaneously form rivets on different planes.
“This application presented us with three major challenges, beginning with parts that vary widely in material thickness,” explains Wright. “In addition, the machine sometimes has to form three or four rivets into a countersink on both sides of the part, or form three rivets on one plane and a fourth rivet on a different plane.”
Looking for a reprint of this article?
From high-res PDFs to custom plaques, order your copy today!